Introduction
A Common Product profile (CP) is a type of data record originally developed as part of a collaboration called the Quartz Project and now maintained by Habitable. The profiles are not specific to any manufacturer, but list the substances that are most commonly present in a product type as well as their associated human and environmental health hazards. Common Product profiles in Pharos may include original records developed by Habitable through the Quartz Project as well as new records since created by Habitable alone.
The purpose of this Common Products database is to be an educational and actionable source of information for those who are looking to more deeply understand the common human and environmental health implications of a variety of building products on the market.
Disclaimer
Common Product profiles are common representations of building products based on numerous respected sources—including specific product literature, transparency documents, trade association data, industry standards, and patents. These profiles are not endorsed by product manufacturers. Real manufactured products can vary widely between manufacturers with respect to product formulations. Common Product data is based on publicly available information at the time of the research and is best used as benchmarks to represent generic products and should not be used as a replacement for specific manufacturer literature and technical documentation. Only a fraction of product categories have been reviewed and included in the current database. If there is a product you would like to see in the database, please write to us at research@habitablefuture.org.
The methodology by which discrete quantities, components, and chemicals have been included in each profile is documented below. Our methodology is rigorous, consistent, and most importantly, transparent.
Investigating and Creating Common Product Profiles
Each Common Product profile (CP) includes the following components:
- A definition that describes the generic, non-manufacturer-specific composition and use of the product.
- A Health Hazard Screening that describes the human and environmental health impacts of the product’s composition, based on Habitable’s Pharos database.
- A list of all potential content that may be used in the product type as identified in the CP research.
- Process Chemistry research from the Pharos database and additional residuals and impurities as documented in the CP research.
Common Product Definition
Every Common Product profile includes the following fields:
| Part | Description |
|---|---|
| Name | The most common way of referring to the CP |
| Description | A summary of the product information, such as the composition, how the product is made and/or installed, key environmental or human health attributes, and/or any assumptions made in defining the CP, as well as references to relevant standards (ASTM, ANSI, etc.) used in determining the boundaries of the product’s content. If an older version of the CP exists, the description also contains a link to the previous version. |
| Common Contents | A functional list of the product’s contents (including residuals or impurities when they are known content of the finished product), with the associated information when applicable: CASRN, percent weight within overall product, function or role within the product, sources (citations), and additional notes. |
| All Contents | A list of both common content and additional less common content that may be present in the product type, with the associated information when applicable: CASRN, function or role within the product, sources (citations), and additional notes. This represents all potential content identified in our research, but does not necessarily include all chemicals and materials that may be used by all manufacturers. |
| Process Chemistry |
Chemicals used or created during manufacturing based on Pharos team research. Information is provided in two sections:
|
Sources of Information
A combination of publicly available documents are used to inform the composition of each Common Product. These documents include, but are not limited to, trade association documents, Environmental Product Declarations (EPDs), Health Product Declarations (HPDs), patents, Safety Data Sheets (SDSs), technical product documents, and documents provided by government, academic, and other authoritative institutions. Because product compositions change frequently, recent sources are preferred over older sources. When no CASRN for a particular chemical is provided in a source, Pharos is used to identify CASRN based on chemical names. Pharos is also used to identify the primary CASRN for a chemical substance that may have multiple CASRNs associated.
Investigating Product Composition
The Common Product database contains a “generic” composition for each CP, as opposed to a manufacturer-specific composition. The most common material or substance for a given function is included in the “Common Contents” (unless multiple substances per function are found to be common), and the proportional allocation of each material or substance is based upon actual formulations in the marketplace to ensure the “generic” compositions represent concentrations found in functional products.
The vocabulary used for describing different components of a Common Product are consistent with terms used in existing standards and assessments, as identified by the Material Health Harmonization Task Group. Read more about their findings in their Material Health Evaluation Programs Harmonization Update report (April 2015).
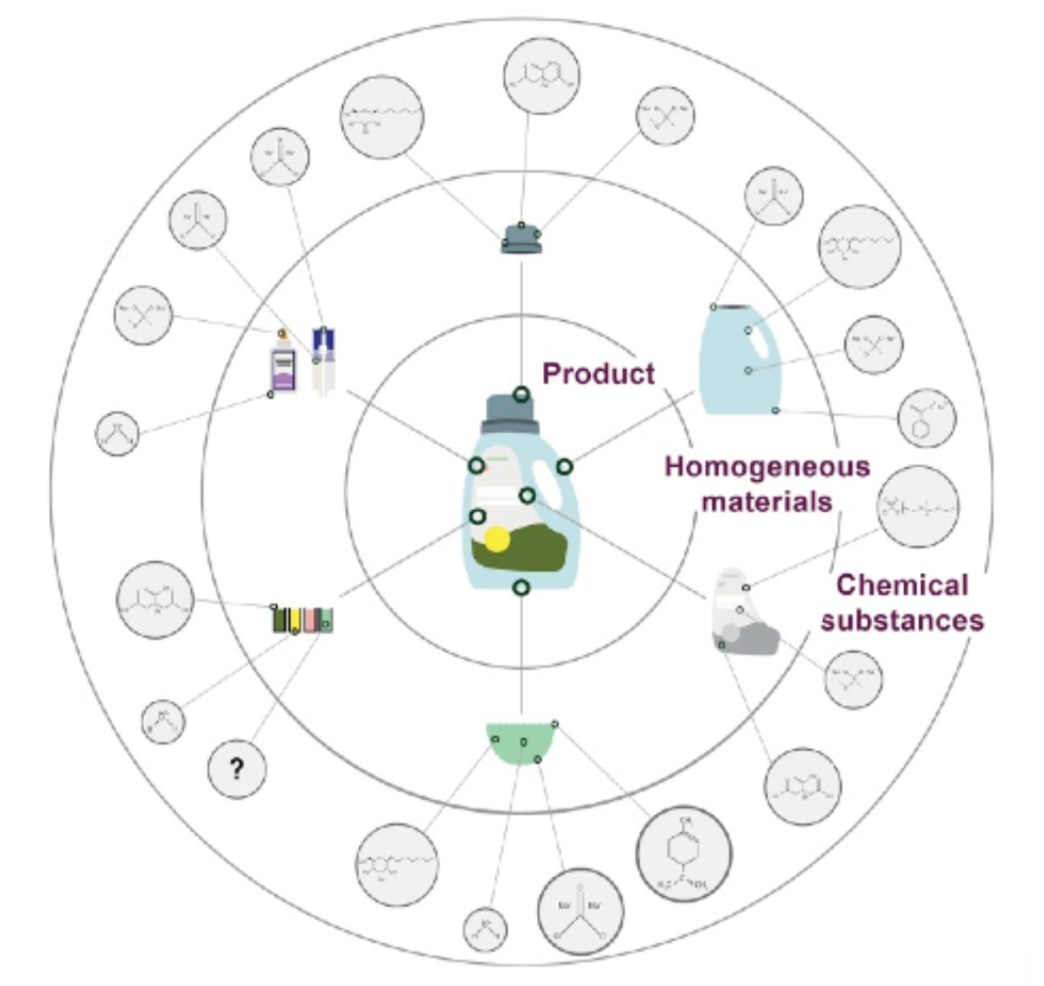
Disclosure
Contents are included in the Common Product profile if they meet the following disclosure thresholds:
- Homogeneous materials are included if present in quantities ≥ 0.01% (100 ppm) of the overall product mass AND cited in two or more sources. If a material is listed in a trade association document or other source that represents more than one manufacturer, the material is eligible for inclusion in the Common Product profile without additional sources.
- Chemical substances are included if present in quantities ≥ 0.01% (100 ppm) of the material’s mass. The same sourcing requirements apply as for homogeneous materials.
- Contents below these thresholds are included if they are hazardous AND either:
- Exceed an established regulatory or other authoritative threshold for presenting a health hazard OR
- Are asthmagens (respiratory health endpoint), carcinogens, mutagens, reproductive or developmental toxicants, endocrine disruptors, or persistent bioaccumulative toxicants (PBT) OR
- Contain impurities that meet the above hazard requirements. For CPs completed prior to 2020, only asthmagens, carcinogens, and PBTs were considered non-threshold substances.
Quantification
The mass percentage of each material or substance is derived by taking a median of the quantities identified across all sources. If the sum of the median percentages does not equal 100%, the percentages are normalized to total 100%, keeping the individual substance percentages within the range reported in the literature.
State
Composition is determined for each product based on its state as delivered to the job site; for example, paint applied on-site is characterized in its liquid state.
Recycled Content CPs
Several CPs have been generated to describe recycled content. For these records, the content represents the recycled material before it is incorporated into a product. Because recycled materials can be highly variable, all content with a minimum of two sources or one authoritative source is listed as common content, and the maximum weight percentages identified are reported instead of the median. Notes may be included to indicate the source(s) of the chemical content when it has been identified.
Capturing High Concern Impurities
The CPs also capture unintentional content when appropriate, as dictated by the following methodology:
Identification
All impurities, including residuals from processing and contaminants from raw materials, are identified using Process Chemistry Research as reported in Pharos or other sources. The Process Chemistry Research looks at substances used in or produced during the manufacturing process, including reactants, catalysts, solvents, by-products, and process aids, as well as contaminants or pollutants commonly found in the materials or substances.
Disclosure
Process chemistry identified in the Pharos database for chemicals that are listed as common content is reported in the “Process Chemistry” tab of the CP. Additional process chemistry identified in the CP research that is related to the relevant product type, but not to any specific chemical, may also be reported here. Residuals or impurities with sufficient data to indicate they are present in the finished product and can be quantified are listed as content (ex. limestone commonly contains quartz as an impurity, so products containing limestone will commonly contain quartz).
Quantification
Mass percentages for impurities within the product are quantified if data is available. Quantities come from (a) aggregated data provided by industry trade associations or government authorities, or (b) a minimum of two independent sources. Impurities that are common and quantifiable are listed in the common content. Impurities that cannot be quantified are included in the process chemistry and the mass percentage is listed as “unknown”. Once quantified, the same threshold rules apply for the inclusion of impurities as for the intentional content detailed above.
Lack of Disclosure
Sometimes publicly available sources are insufficient for identifying discrete materials or substances beyond a general description. In such cases, a proxy material or substance is reported that matches the function of the unspecified content. For example, public information about coatings applied to the interior of sprinkler pipes describe them as “epoxy” with no further specificity. A CP for a general epoxy high-performance coating was used as the proxy for what might be used specifically in sprinkler pipes.
Polymers
Lack of disclosure often occurs with polymers, whose identities are sometimes held as proprietary and only referenced using a general name within the product literature. In such cases, the identity of a given polymer is determined from patent information if possible. Patents often provide a formulation of ingredients that are reacted to form the finished polymer and can be used to identify common reactants. These common reactants are listed as impurities if they pose a hazard not already captured by the polymer. In addition, if the mass percentage of a polymer within a product is not available, it is estimated by adding the mass percentages of its reactants (such as monomers and crosslinkers) within the product. If the polymer’s specific name or CASRN cannot be identified, it is listed by its general name without a CASRN and “unknown” health hazards.
When no common reactants are known but a representative polymer is identified (i.e., no specific polymer is identified as common, but one source references the CASRN), the identified polymer is included as a representative polymer.
When no common reactants are known and no representative CASRN is identified, the polymer is listed by its general name without a CASRN. No residual information is included unless it is available from product literature. The health hazards are “unknown”.
Source Documentation
All content included in the CP is appropriately cited with a list of sources from which the information was derived. This includes sources used to determine the common substance or material as well as percentages and functional use. Additional sources that contributed to defining any other part of the profile are also cited.
All Contents
Throughout the CP research process, Habitable identifies a range of content that may be present in a product type. This includes chemicals for functions that were not found to be common and alternative chemicals for common functions in the CP. This additional information is included in the Common Product record in Pharos in the “All Contents” tab. This is not necessarily representative of all chemicals and materials that may be used by all manufacturers and should not be used as a replacement for a specific manufacturer's product disclosure.
Common Product Health Hazard Screening
About Health Hazard Screening
A variety of state, national, and international governmental bodies and non-governmental organizations (NGOs) maintain authoritative chemical hazard lists. These are lists of substances for which an authoritative body of scientists has undertaken a systematic review of scientific evidence and categorized the substances as having an association with a specific health hazard. There is currently no single, comprehensive authoritative list or database that assesses and rates all chemicals across all human health hazard endpoints. Pharos begins to address this problem by combining many single hazard endpoint lists into one combined database that provides a view across multiple endpoints.
Health Hazard Screening of Common Products
Health hazard data from Pharos is used to screen the chemical substances within the CPs to determine if the substances have been associated with a health concern by an authoritative body. The GreenScreen® for Safer Chemicals Benchmark or List Translator score is displayed next to each chemical in the CP profile. Additional hazard data from dozens of other authoritative hazard lists from Pharos can be accessed by clicking on the chemical itself. The screenings do not include a risk or exposure assessment. If, however, the hazard screening designates the hazard as occupational, this is indicated.
See the system description for Pharos for more information on hazard flags and health hazard endpoints as well as the authoritative lists and governing bodies responsible for hazard warnings in the CPs.
Glossary
- CASRN
- A Chemical Abstract Services Registry Number is a unique identifier assigned by the Chemical Abstract Service of the American Chemical Society to uniquely identify chemical elements, compounds, polymers, and other materials and mixtures. Frequently used in Safety Data Sheets (SDSs). Also known as a “CAS number”.
- Chemical Substance or Substance
- A substance of fixed composition, characterized by its molecular structure(s), which typically has an associated CASRN
- Common Product profile (CP)
- A profile of a generic, non-manufacturer-specific product that contains: 1) definition and composition based on curated, public, technical documents, and 2) health hazard screen data based on the Pharos database.
- Contents
- A general umbrella term for everything in a product or part (homogenous materials and/or chemical substances).
- GreenScreen List Translator™
- The GreenScreen List Translator maps authoritative and screening hazard lists, including Globally Harmonized System (GHS) country classifications, to GreenScreen hazard classifications. The GreenScreen List Translator can facilitate screening and classification of chemicals based on national and international regulatory sources, influential NGO lists of chemicals of concern, deliberations from authoritative scientific bodies, European Risk and Hazard Phrases, and government chemical classifications using the GHS of Classification and Labelling.
- Health Hazard Screening
- Review of health hazards associated with a substance based on authoritative chemical hazard lists
- Homogeneous Material or Material
- A uniform solid, liquid, or gas composed of one or more substances that cannot be mechanically disjointed, in principle.
- Human Health Endpoint
- Disease symptom or related marker of a health impact on a human or other being, e.g., cancer or reproductive toxicity.
- Impurity (Residual or Contaminant)
- An unintended constituent present in a material/mixture as manufactured. It may originate from the starting materials or be the result of secondary or incomplete reactions during the manufacture process. While it is present in the final material/mixture, it was not intentionally added.
- Material Health Harmonization Task Group
- The Material Health Harmonization Task Group (HTG) includes Business and Institutional Furniture Manufacturers Association (BIFMA), Cradle to Cradle Products Innovation Institute (C2CPII), GreenScreen/Clean Production Action (CPA), Habitable, and the Health Product Declaration Collaborative (HPDC). This group was funded by the US Green Building Council to map the similarities and differences between the various programs and work towards synergies through inter-program harmonization and data sharing.
- Pharos
- A database for identifying health and environmental hazards associated with chemicals, materials, and building products, developed by Habitable.
- Product
- A finished good composed of parts and/or homogeneous materials. A product may function as part of another product. A product may be made of one or more homogeneous materials, which are composed of chemical substances.
- Quartz Project
- The Quartz Project was an open data initiative promoting the transparency of building products. The project, a collaboration between Habitable (then Healthy Building Network), Google, Flux, and thinkstep, resulted in an open database of 102 Common Product profiles including common content, health hazards, and LCA data. This database represents a snapshot of information as of 2015 and is no longer being maintained or updated.